Women In Challenging Modern Society



The Future Is Here
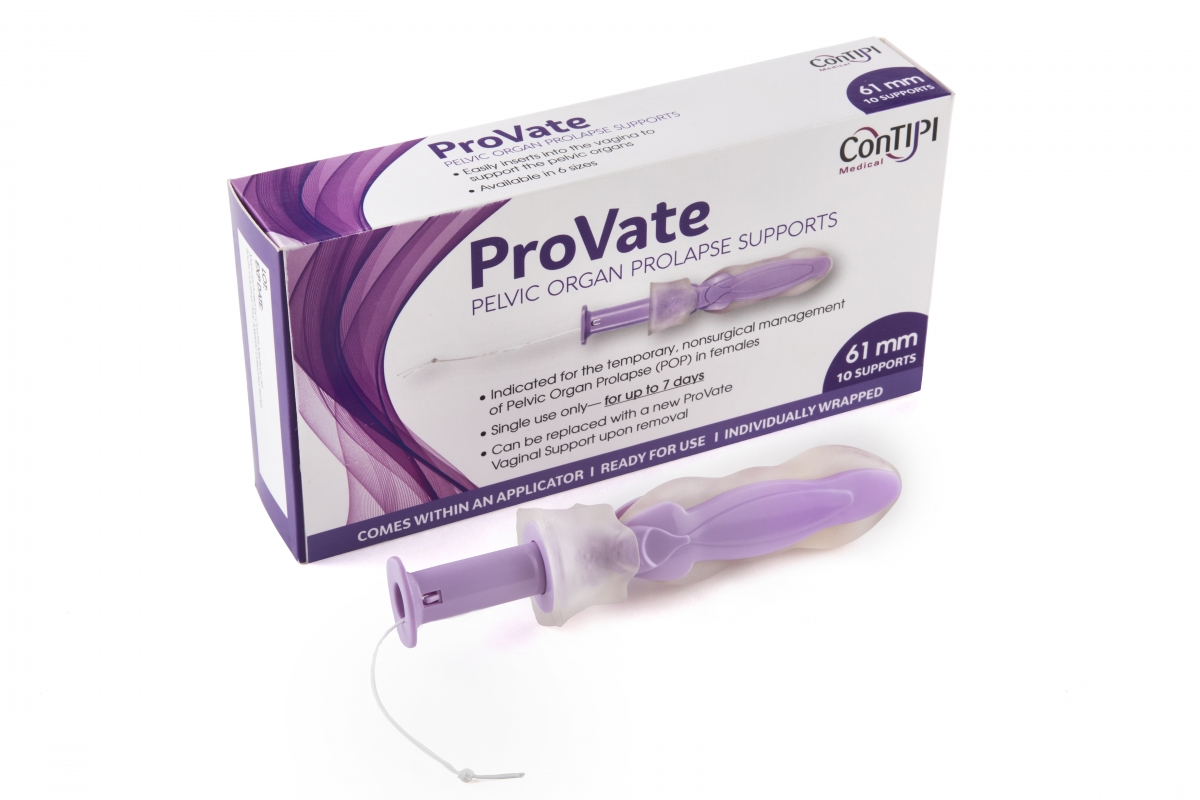
ConTIPI Medical provides cutting edge non-surgical and disposable vaginal solutions for women with various Pelvic Floor Disorders (PFD’s). These solutions are all vaginal devices which follow a cutting edge concept. They all come ready for use, inserted vaginally in small dimensions inside an applicator. Within the vagina they open and provide support to predefined specific sites along vaginal walls. By the end of usage, devices are removed by a pull of a string, which also causes them to considerably diminish in size, for disposal. Previous device, for the management of Stress Urinary Incontinence (SUI), was sold to Kimberly Clark Worldwide Inc, and is marketed under its Poise® brand (https://www.impressapro.com). ConTIPI Medical’s ProVate Device, for the non-surgical management of Pelvic Organ Prolapse (POP) has a 510(k) clearance from FDA for marketing in the United States, and a CE Mark for marketing in the EU.
Our Capabilities
Medical knowledge by experienced urogynecologist
strategies are backed by well recognized business experts
Extensive and strong Intellectual Property
Creative R&D team
Idea conception and brainstorming are done internally
Regulatory affairs are managed in-house
Experience in conducting clinical studies from early stages
In-house production and assembly abilities
POP In The US
0
Women with POP
0
women consult on POP with their doctor
0
women are eventually treated for POP