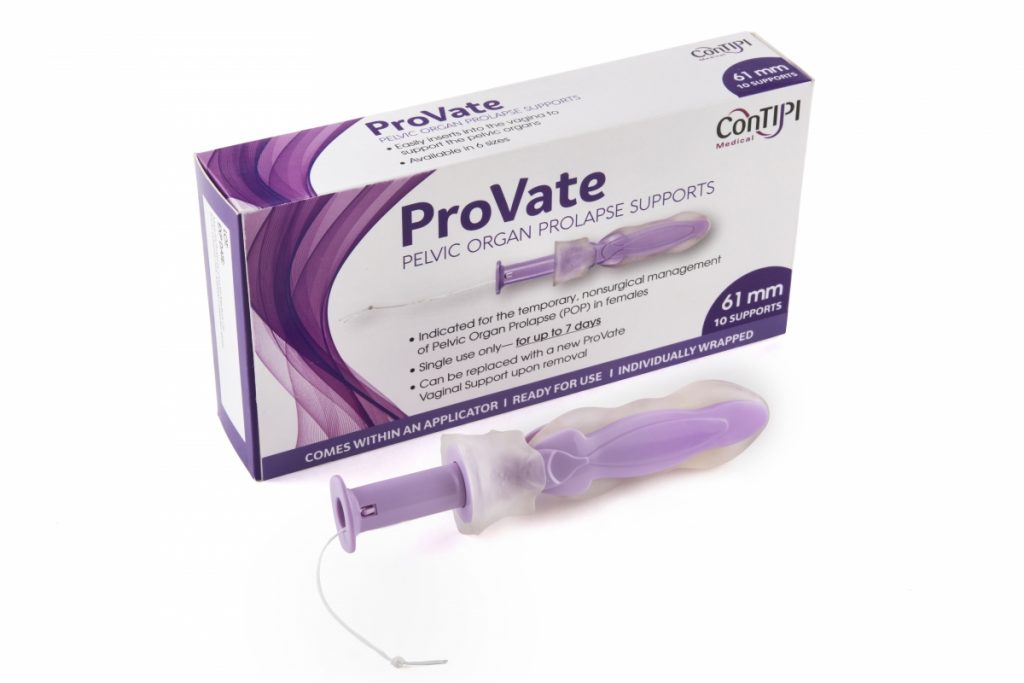
ProVate Device
Most women with POP will only require non-invasive management. Existing pessaries are associated with high discontinuation rate, and with high rate of reluctance to use a reusable device which is difficult for most users to insert and remove.
The ProVate Device is a vaginal ring pessary designed to perform exactly as the existing ring pessary, while reducing or even eliminating some of the problems mentioned above. The device comes ready for use in small dimensions (compacted mode) within a disposable applicator. The applicator allows for a smooth and comfortable insertion into the vagina. When the plunger of the applicator is depressed, the device becomes fully deployed, restoring its predefined size (deployed mode), and separates from the applicator which is then removed from the vagina for disposal.
It is made of a flexible skeleton covered by a soft elastomer. Once deployed within the vagina, as with other ting pessaries, the ProVate support distends lateral vaginal walls aside, mechanically prevents cervical/vault descent, and with its central piece – blocks further descent of the anterior/posterior walls within the hollow of the ring. The device is provided in 6 sizes, to accommodate various vaginal dimensions.
Following size fitting and initial training by a medical practitioner at the clinic, the ProVate Device can be self-inserted and removed by the user at her home environment (physician directed home-use, with no need for cleaning and replacement by a medical practitioner), with only minimal touch of her genital area. In its mode of insertion and removal, the ProVate Device resembles the use of a regular menstrual tampon, being inserted vaginally with an applicator, and removed by a pull of a string, for disposal.
Following usage of up to 7 days, the user pulls the removal string, and the device collapses into its narrow pre-insertion dimensions (compact mode) for an easy and comfortable removal out of the vagina for disposal.
The ProVate Device and the research surrounding it were awarded the Best Novel Therapy presentation during the American Urogynecological Society (AUGS) meeting, Providence RI, in October 2017.

ConTIPI Medical’s ProVate Device, for the non-surgical management of Pelvic Organ Prolapse (POP) has a 510(k) clearance from FDA for marketing in the United States, and a CE Mark for marketing in the EU.