ConTIPI Medical, and its associate company ConTIPI Limited, both located at the Caesarea Industrial Park in Israel, provide non-surgical and disposable vaginal medical device solutions for women with various Pelvic Floor Disorders (PFD’s). Each device comes ready for use within a wrapper for the woman to use in the comfort of her own home on her schedule. The devices are inserted vaginally in small dimensions inside an applicator. Within the vagina they open and provide support to pre-defined specific sites along vaginal walls. By the end of usage, devices are removed by a pull of a string, which also causes them to considerably diminish in size, for disposal. Such devices allow women to take control over their medical problem, and use them in privacy and their preferred time.
About
The Vision
ConTIPI’s concept has already proved itself, brought from a concept into a mature product in the market. The Impressa Device for Stress Urinary Incontinence, bought by Kimberly Clark Worldwide Inc, is marketed by Kimberly Clark Inc. under its Poise® brand (https://www.impressapro.com).
ConTIPI Medical’s ProVate Device, for the non-surgical management of Pelvic Organ Prolapse (POP) has a 510(k) clearance from FDA for marketing in the United States, and a CE Mark for marketing in the EU. ConTIPI Medical intends to develop more solutions for other Pelvic Floor Disorders in women, following its unique concept.


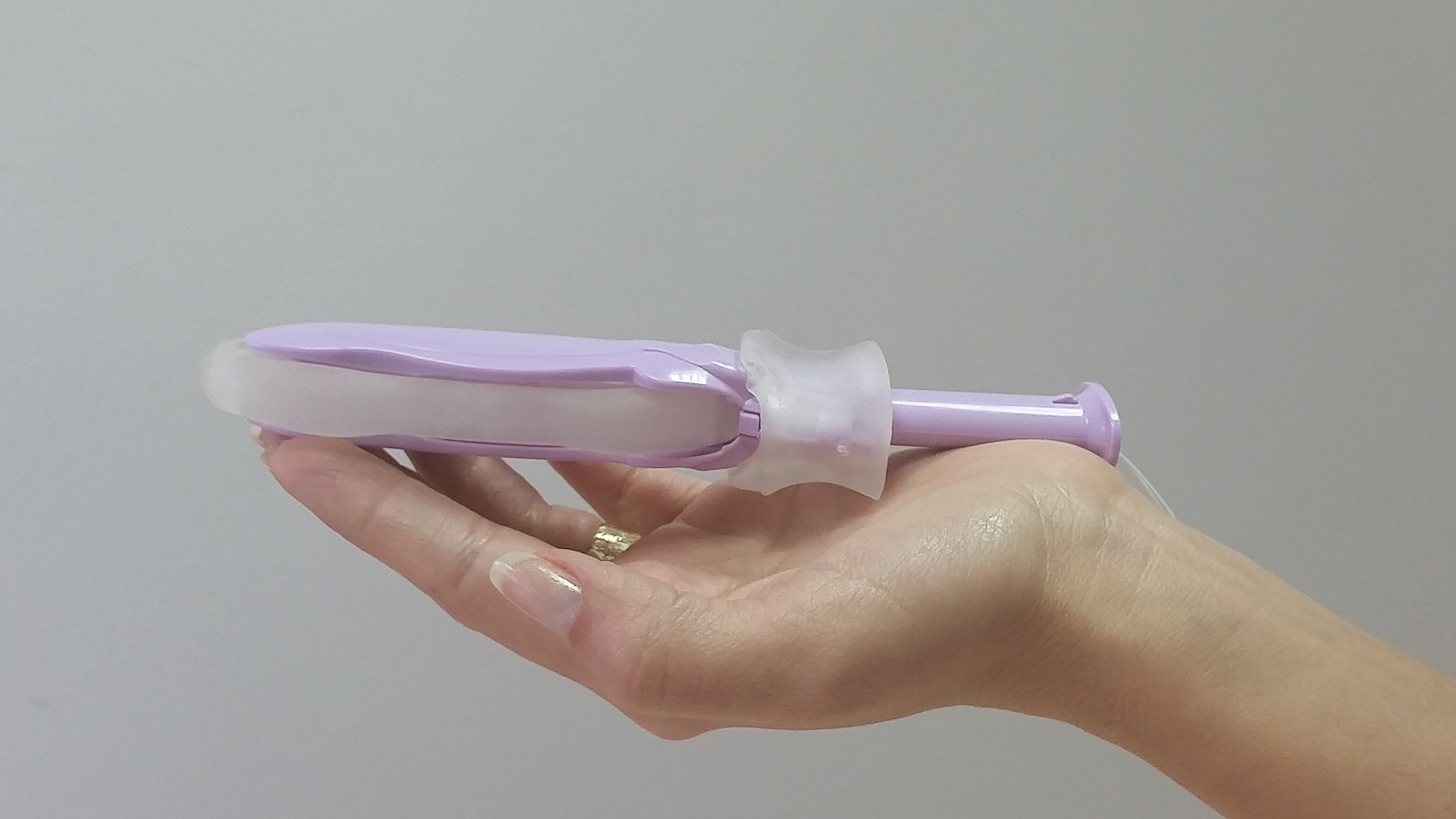
Capabilities
As a dedicated Research and Development (R&D) company, the entire development of its products is conducted within the company
The Management
Advisory Board
Regulatory Affairs and Clinical Research at ConTIPI Medical
Within an R&D company, regulatory affairs and clinical research play an important role in both development of the products and in post-development clinical assessment.
ConTIPI Medical has extensive in-house capabilities both in regulatory affairs and in clinical studies, conducting its clinical trials either by itself or with a CRO, in Israel or abroad.
Regulatory affairs and clinical research at ConTIPI is headed by Tsvia Erlich and is backed by Hogan Lovells in Washington DC, USA
Quality Assurance
ConTIPI Medical Ltd. is certified for ISO 13485 since 2006.
The quality system complies with FDA QSR (21CFR Part 820)
The company is CE certified according to the medical device Directive 93/42/EEC by MDC CE0483, Germany.
The company is committed to continuous innovations and improvements of its production facilities, product quality, meeting customer needs and applicable regulatory requirements.
The development process is done according to Design Control procedures of ISO 13485 and FDA QSR (21CFR Part 820).




